The Treatment of Saphenous Vein Occlusion by Endovenous Laser Ablation (EVLA) with a 1064 nm Laser
The Treatment of Saphenous Vein Occlusion by Endovenous Laser Ablation (EVLA) with a 1064 nm Laser
1064 nm diode laser is an effective and safe treatment modality for varicose veins, Due to higher patient satisfaction, shorter recovery times, lower cost, and ease of operation Endovenous Laser Ablation (EVLA) of the great and/or short saphenous vein has become the treatment of choice for varicose veins. EVLA shows minimal side effects in comparison with other surgical methods; patients can walk immediately after surgery and recovery times are short. The EVLA procedure can be performed
in an outpatient setting and usually, only local anesthesia is required.
The principle mechanism of EVLA therapy is ablation and photocoagulation of the vein interior by laser- induced thermal effects. In brief, EVLA is accomplished by inserting an optical fiber just below the saphenofemoral junction (SFJ) through the great saphenous vein (GSV) and delivering laser energy through the fiber as it is withdrawn down the GSV. During fiber withdrawal the vein wall is irreversibly
destroyed and the vein is occluded. A full procedure is presented below in the Surgical Protocol section. A similar procedure can be followed for the small
saphenous vein (SSV), in which the tip of the fiber is positioned at the saphenopoliteal junction. Other superficial veins and perforators of suitable
length and diameter can also be treated.
The primary parameters governing EVLA are the laser source wavelength, pulse form and power, and the optical fiber withdrawal rate.
Wavelength Considerations in EVLA
To develop the necessary heat for effective vein wall destruction and occlusion, the target tissue must absorb laser light. Different chromophores in the target tissue absorb laser light with different efficiency at different wavelengths. For successful EVLA treatment, hemoglobin and water in the blood and vein walls must absorb laser light. Water and hemoglobin absorb well across a wide range of wavelengths and many wavelengths have been successfully used in EVLA; 810nm, 940nm,
980nm, 1064nm, 1320nm, and 1470 nm. In the case study presented in this paper a 1064nm wavelength was used for all EVLA treatments.
Laser Power Considerations in EVLA Delivering a large amount of energy in a very short time is important to the proper and efficacious administration of EVLA. In this study, laser power was the only parameter that changed between treatment groups. It was increased from 15-18W to 25W. Recent research suggests that using higher laser powers improves treatment efficacy. Energy delivery is usually reported in units of joules per centimeter of the vein. This is referred to as linear endovenous energy density or LEED.
Pulse Form Considerations in EVLA
In DIMED diode lasers, which operate continuously, single pulsed and repeated pulsed mode. Lasers fire many times per second. Each pulse itself has a duration or
period; during that period light, and therefore energy, is delivered through the optical fiber-end. In some laser systems the pulse power can vary significantly
during the duration of the pulse, resulting in suboptimal energy delivery and thus reduced efficacy in vein destruction and occlusion. In this case study, the power of each laser pulse was constant during the entire pulse duration, enabling well-
controlled laser-tissue interaction. This was enabled by proprietary, DIMED laser
technology.
Withdrawal Rate Considerations in EVLA The withdrawal rate of the optical fiber affects the amount of energy delivered to the vein wall. The best treatment results are achieved when the optimal amount of energy is uniformly distributed along the
length of the vein. The diameter of the vein wall changes along the vein length, from 10-20mm at the SFJ to 2-3mm at the entry point, so to deliver constant energy per vein wall surface area, either the withdrawal rate or the laser energy must vary. Some manufacturers promote motorized withdrawal systems that provide a constant withdrawal rate to perform EVLA procedures. Yet these solutions cannot provide uniform energy distribution if the laser power is also held constant. Therefore most practitioners prefer to manually control the withdrawal rate while the laser delivers constant energy; withdrawing the fiber slowly in wide sections of the vein and faster in narrow sections. Withdrawal rate was manually controlled during this study (see the Surgical Protocol section).
Surgery was performed according to the following steps. First, the patients were premedicated with a midazolam (Dormicum, Roche) sedative and 125 mg
diklofenak (Naklofen, Krka) analgesic. Vein assessment and mapping by color-flow duplex scan was performed and a decision was made about the point of vein cannulation. The patient’s leg was cleaned, draped, and a sterile operating field was
prepared. A layer of gel was applied to the US probe and the probe was then covered with a watertight cover.
The patient was placed in the anti-Trendelenburg position on the table. Local intradermal anesthesia was infiltrated through a 27G needle at the point of
percutaneous insertion. Care was taken so that the needle did not touch the vein. The vein was then punctured under ultrasound (US) control with a 19G needle at the selected entry point. A 0.035'' J tipped guide wire was introduced into the vein. The guide was slid to the SFJ, aided by duplex monitoring, and then positioned at the SFJ. A stab incision with a blade 11 knife was made at the entry point of the guide wire. A dilator was introduced over the guide wire.
Before inserting the laser fiber and catheter assembly into the vein, the optimal laser fiber length was determined outside of the body. The laser fiber was introduced into the catheter and positioned so as to protrude 2.5cm from the distal end of the catheter. The stopper at the proximal end of the catheter was firmly tightened onto the fiber. The fiber (with properly positioned and secured stopper) was removed from the catheter. This step ensured that the fiber protrudes correctly from the catheter when the catheter is in the vein. The dilator on the guide wire was extracted. The catheter was passed over the guide wire to the desired position at the SFJ. Once the catheter was in position the guide wire was removed, leaving the catheter
inside the vein. The fiber was introduced into the catheter and locked into its predefined position with the stopper. Further fine adjustments were made to the catheter and fiber tip position under US control. The laser fiber was then connected to the laser system.
No problems were reported by any of the patients, except for mild skin irritation and ecchymosis. No deep vein thrombosis was recorded. The micro- phlebectomies usually showed transient and insignificant bruising. During follow-up appointments
patients were checked with US for signs of full or partial vein recanalization. Six months after the surgery, 94% of the veins in the 15-18W treatment group and 99.2% of the veins in the 25W treatment group remained occluded. After 1 year, 88.2% of the veins in the 15-18W and 98.5% in the 25W treatment group remained occluded. All patients, even those whose veins were not fully occluded, reported being satisfied with treatment.
The results seen in this clinical study are in-line with results from Proebstle et al , who saw statistically significant differences in occlusion rate (9.7% more) between 15W and 30W at a wavelength of 940nm. Yet the same study demonstrated a statistically significant effect on side effects between three cohorts; A (940nm, 15W), B (940nm, 30W) and C (1320nm, 8W). Patients in cohort C showed less ecchymosis and felt less pain than patients in the other cohorts. Because of differences in both wavelength and power, it is difficult to directly apply these findings to treatments
at 1064nm and 25W. In our study, using the same 1064nm wavelength in both patient groups, we observed no increase in side effects for procedures administered at 25W versus those at 15-18W.
1064 nm diode laser is an effective and safe treatment modality for varicose veins, Due to higher patient satisfaction, shorter recovery times, lower cost, and ease of operation Endovenous Laser Ablation (EVLA) of the great and/or short saphenous vein has become the treatment of choice for varicose veins. EVLA shows minimal side effects in comparison with other surgical methods; patients can walk immediately after surgery and recovery times are short. The EVLA procedure can be performed
in an outpatient setting and usually, only local anesthesia is required.
The principle mechanism of EVLA therapy is ablation and photocoagulation of the vein interior by laser- induced thermal effects. In brief, EVLA is accomplished by inserting an optical fiber just below the saphenofemoral junction (SFJ) through the great saphenous vein (GSV) and delivering laser energy through the fiber as it is withdrawn down the GSV. During fiber withdrawal the vein wall is irreversibly
destroyed and the vein is occluded. A full procedure is presented below in the Surgical Protocol section. A similar procedure can be followed for the small
saphenous vein (SSV), in which the tip of the fiber is positioned at the saphenopoliteal junction. Other superficial veins and perforators of suitable
length and diameter can also be treated.
The primary parameters governing EVLA are the laser source wavelength, pulse form and power, and the optical fiber withdrawal rate.
Wavelength Considerations in EVLA
To develop the necessary heat for effective vein wall destruction and occlusion, the target tissue must absorb laser light. Different chromophores in the target tissue absorb laser light with different efficiency at different wavelengths. For successful EVLA treatment, hemoglobin and water in the blood and vein walls must absorb laser light. Water and hemoglobin absorb well across a wide range of wavelengths and many wavelengths have been successfully used in EVLA; 810nm, 940nm,
980nm, 1064nm, 1320nm, and 1470 nm. In the case study presented in this paper a 1064nm wavelength was used for all EVLA treatments.
Laser Power Considerations in EVLA Delivering a large amount of energy in a very short time is important to the proper and efficacious administration of EVLA. In this study, laser power was the only parameter that changed between treatment groups. It was increased from 15-18W to 25W. Recent research suggests that using higher laser powers improves treatment efficacy. Energy delivery is usually reported in units of joules per centimeter of the vein. This is referred to as linear endovenous energy density or LEED.
Pulse Form Considerations in EVLA
In DIMED diode lasers, which operate continuously, single pulsed and repeated pulsed mode. Lasers fire many times per second. Each pulse itself has a duration or
period; during that period light, and therefore energy, is delivered through the optical fiber-end. In some laser systems the pulse power can vary significantly
during the duration of the pulse, resulting in suboptimal energy delivery and thus reduced efficacy in vein destruction and occlusion. In this case study, the power of each laser pulse was constant during the entire pulse duration, enabling well-
controlled laser-tissue interaction. This was enabled by proprietary, DIMED laser
technology.
Withdrawal Rate Considerations in EVLA The withdrawal rate of the optical fiber affects the amount of energy delivered to the vein wall. The best treatment results are achieved when the optimal amount of energy is uniformly distributed along the
length of the vein. The diameter of the vein wall changes along the vein length, from 10-20mm at the SFJ to 2-3mm at the entry point, so to deliver constant energy per vein wall surface area, either the withdrawal rate or the laser energy must vary. Some manufacturers promote motorized withdrawal systems that provide a constant withdrawal rate to perform EVLA procedures. Yet these solutions cannot provide uniform energy distribution if the laser power is also held constant. Therefore most practitioners prefer to manually control the withdrawal rate while the laser delivers constant energy; withdrawing the fiber slowly in wide sections of the vein and faster in narrow sections. Withdrawal rate was manually controlled during this study (see the Surgical Protocol section).
Surgery was performed according to the following steps. First, the patients were premedicated with a midazolam (Dormicum, Roche) sedative and 125 mg
diklofenak (Naklofen, Krka) analgesic. Vein assessment and mapping by color-flow duplex scan was performed and a decision was made about the point of vein cannulation. The patient’s leg was cleaned, draped, and a sterile operating field was
prepared. A layer of gel was applied to the US probe and the probe was then covered with a watertight cover.
The patient was placed in the anti-Trendelenburg position on the table. Local intradermal anesthesia was infiltrated through a 27G needle at the point of
percutaneous insertion. Care was taken so that the needle did not touch the vein. The vein was then punctured under ultrasound (US) control with a 19G needle at the selected entry point. A 0.035'' J tipped guide wire was introduced into the vein. The guide was slid to the SFJ, aided by duplex monitoring, and then positioned at the SFJ. A stab incision with a blade 11 knife was made at the entry point of the guide wire. A dilator was introduced over the guide wire.
Before inserting the laser fiber and catheter assembly into the vein, the optimal laser fiber length was determined outside of the body. The laser fiber was introduced into the catheter and positioned so as to protrude 2.5cm from the distal end of the catheter. The stopper at the proximal end of the catheter was firmly tightened onto the fiber. The fiber (with properly positioned and secured stopper) was removed from the catheter. This step ensured that the fiber protrudes correctly from the catheter when the catheter is in the vein. The dilator on the guide wire was extracted. The catheter was passed over the guide wire to the desired position at the SFJ. Once the catheter was in position the guide wire was removed, leaving the catheter
inside the vein. The fiber was introduced into the catheter and locked into its predefined position with the stopper. Further fine adjustments were made to the catheter and fiber tip position under US control. The laser fiber was then connected to the laser system.
The proper position of the fiber inside GSV, below
SFJ and just below the Epigastric vein
Tumescent anaesthesia was delivered through a syringe or by special pump. When administered by syringe the anaesthetic solution consisted of 20ml of 2% lignocain (Xylocain) diluted in 100ml of saline and a 4ml of 1N solution of sodium bicarbonate. When applying anaesthesia with a special pump the anaesthetic solution consisted of 40ml of 2% lignocain (Xylocain) diluted in 500ml of saline with the addition of 8 ml of 1N solution sodium bicarbonate and 1ml of 0.1% Epinephrine.
The laser was activated and during lasing the fiber was slowly withdrawn. The first 7 cm of extraction were US controlled. Every 10 cm the amount of delivered energy was checked. Laser energy was administered according to the generally accepted rule that LEED (J/cm) is 10x the vein diameter (mm). For the first 7cm, energy by 50% in excess of the value indicated by this rule was administered. The delivery of laser light was stopped 2cm from the surgical entry point. After the laser procedure was complete micro- phlebectomies were performed to remove smaller superficial varicosities. Sutures and steri-strips were applied to the incisions. Class II medical compression stockings were given to the patients to wear for 4 weeks after the treatment. Follow-up appointments were done 1 week, 4 weeks, 6 months and 1 year after the treatment session.No problems were reported by any of the patients, except for mild skin irritation and ecchymosis. No deep vein thrombosis was recorded. The micro- phlebectomies usually showed transient and insignificant bruising. During follow-up appointments
patients were checked with US for signs of full or partial vein recanalization. Six months after the surgery, 94% of the veins in the 15-18W treatment group and 99.2% of the veins in the 25W treatment group remained occluded. After 1 year, 88.2% of the veins in the 15-18W and 98.5% in the 25W treatment group remained occluded. All patients, even those whose veins were not fully occluded, reported being satisfied with treatment.
The results seen in this clinical study are in-line with results from Proebstle et al , who saw statistically significant differences in occlusion rate (9.7% more) between 15W and 30W at a wavelength of 940nm. Yet the same study demonstrated a statistically significant effect on side effects between three cohorts; A (940nm, 15W), B (940nm, 30W) and C (1320nm, 8W). Patients in cohort C showed less ecchymosis and felt less pain than patients in the other cohorts. Because of differences in both wavelength and power, it is difficult to directly apply these findings to treatments
at 1064nm and 25W. In our study, using the same 1064nm wavelength in both patient groups, we observed no increase in side effects for procedures administered at 25W versus those at 15-18W.
Transient bruising as a side effect of micro-
phlebectomies performed in the same session with EVLA
at two weeks (a) and two months (b) after the procedure
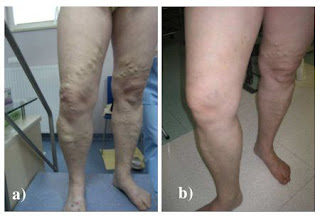
EVLA provides an excellent aesthetic result –
typical patient from this study, before (a) and after (b)
the procedure.
More information about diode laser with 810nm/980nm/1064nm/1470nm/1940nm wavelength please contract:
Wuhan Dimed Laser Technology Co., Ltd






Comments
Post a Comment